Abstract
Introduction: With the availability of multiple therapy classes targeting BCMA for multiple myeloma (MM), data are needed to understand effective treatment (tx) sequencing. CARTITUDE-2 (NCT04133636) is a phase 2, multicohort study evaluating the efficacy and safety of cilta-cel, an anti-BCMA CAR-T therapy, in several MM patient populations. We present updated results with a longer follow-up on cohort C patients with previous exposure to a non-cellular anti-BCMA immunotherapy.
Methods: Cohort C patients had progressive MM after tx with a proteasome inhibitor, immunomodulatory drug, anti-CD38 antibody, and non-cellular BCMA-targeting agent. A single cilta-cel infusion (target dose: 0.75×106 CAR+ viable T cells/kg) was administered 5-7 days post lymphodepletion. The primary endpoint was minimal residual disease (MRD) negativity at 10-5. Secondary endpoints included overall response rate (ORR; assessed by IMWG criteria), duration of response (DOR), and adverse events (AEs).
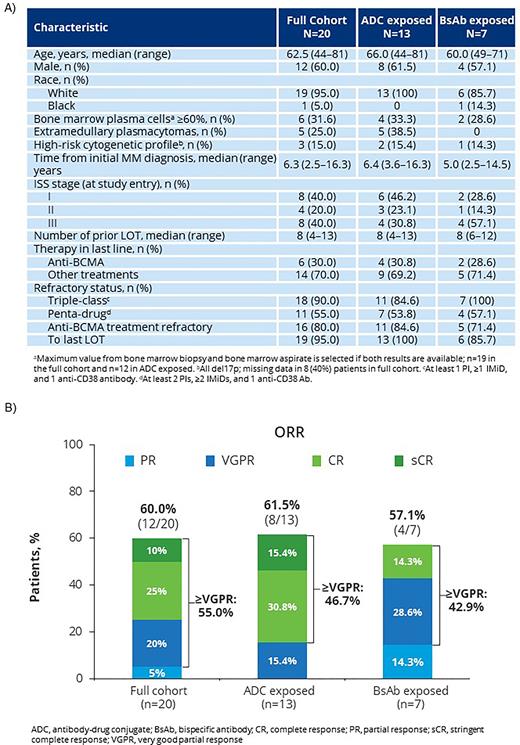
Results: As of June 1, 2022, 20 patients (13-ADC exposed; 7 BsAb exposed; 1 patient in the ADC group also had prior BsAb exposure) were treated with cilta-cel. Four patients did not receive cilta-cel due to either low cellular yield (n=2, 1 patient in each group) or death due to progressive disease (PD) prior to dosing (n=2, 1 patient in each group). Six patients (30%) received anti-BCMA tx as their last line of therapy (LOT; n=4 ADC, n=2 BsAb). During prior anti-BCMA tx, best responses included very good partial response (VGPR; 2 patients in ADC group, 1 patient in BsAb group), stringent complete response (sCR, 1 patient in ADC group), and complete response (CR; 1 patient in BsAb group); the rest had best response of stable disease (SD) or PD (1 patient was not evaluable [NE]). At baseline, median age was 62.5 y (range, 44-81), 60% were male, 3 (15%) had high risk cytogenetics (all del17p), and 5 (25%) had baseline extramedullary disease. Patients had received a median of 8 (range, 4-13) prior LOT; 18 (90%) were triple-class refractory, 11 (55%) penta-drug refractory, and 16 (80%) refractory to non-cellular anti-BCMA treatment (Figure 1A). The median time from last anti-BCMA agent to cilta-cel infusion was 195 d (range, 62-749). Median administered dose of cilta-cel was 0.65x106 (range, 0.21x106- 1.11x106) CAR+ viable T cells/kg; 1 patient received a below-target dose. At a median follow-up of 18.0 mo (range, 0.6-22.7), 7 of 10 evaluable patients (70%) were MRD-negative at 10-5 (5 of 7 evaluable patients [71.4%] in the ADC group, and 2 of 3 evaluable patients [66.7%] in the BsAb group). The ORR was 60% (95% CI, 36.1 - 80.9) for the full cohort (61.5% [95% CI, 31.6-86.1] in the ADC group and 57.1% [95% CI, 18.4-90.1] in the BsAb group; Figure 1B). Median DOR (95% CI) was 12.8 mo (7.9-NE) in the full cohort, 12.8 mo (7.9-NE) in the ADC group, and 8.2 mo (4.4-NE) in the BsAb group. Median PFS (95% CI) was 9.1 (1.5-13.8) mo in the full cohort, 9.5 mo (1.0-15.2) in the ADC group, and 5.3 mo (0.6-NE) in the BsAb group. Patients who responded to cilta-cel had a shorter median duration of last anti-BCMA agent exposure (29.5 d, range 1-277) compared with non-responders (63.5 d, range 54-260). Responders also had a longer median time from last anti-BCMA tx exposure to apheresis (161.0 d, range 26-695) than non-responders (56.5 d, range 40-77). The most common AEs were hematologic. CRS occurred in 12 (60%) patients (all grade 1/2), with median time to onset of 7.5 d (range, 2-10) and median duration of 6.0 d (3-10). ICANS occurred in 4 (20%) patients (2 grade 3/4), with median time to onset of 9.0 d (range, 4-13) and median duration of 7.0 d (range, 4-20). ICANS was recovered or resolved in 3 patients. No patient had movement or neurocognitive tx emergent AE/parkinsonism. There were 12 deaths: 8 due to PD, 2 due to COVID-19 pneumonia (not tx related), and 1 each due to subarachnoid hemorrhage (not tx related) and C. difficile colitis (tx related).
Conclusions: Heavily pretreated MM patients with previous exposure to a non-cellular anti-BCMA therapy had favorable responses following cilta-cel. However, depth and DOR appear lower than that seen in anti-BCMA-naïve patients treated with cilta-cel (at 27.7 mos, median DOR was not reached in heavily pre-treated but anti-BCMA naïve CARTITUDE-1 patients). These results may inform tx plans, including sequencing and washout period between BCMA-targeting agents.
Figure 1: (A) Patient Demographics and Disease Characteristics, (B) ORR
Disclosures
Cohen:Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Ichnos, Janssen Oncology, Oncopeptides, Pfizer, Seattle Genetics, Genentech/Roche, AstraZeneca, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline and Novartis: Research Funding; Novartis: Patents & Royalties: CAR T-cells and biomarkers of cytokine-release syndrome. Mateos:Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides, Seagen: Honoraria; Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides: Membership on an entity's Board of Directors or advisory committees. Cohen:Medison: Honoraria; GSK: Honoraria; Takeda: Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Amgen: Honoraria, Research Funding; Neopharm: Honoraria. Rodriguez Otero:BMS: Consultancy; Sanofi: Consultancy, Speakers Bureau; Pfizer: Consultancy; GSK: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; BMS-Celgene: Speakers Bureau; Regeneron Pharmaceuticals, Inc.: Speakers Bureau; Amgen, Sanofi, GSK, Janssen, BMS-Celgene, Regeneron: Speakers Bureau; Janssen, BMS, Sanofi, Pfizer, GSK.: Consultancy; Janssen: Consultancy, Speakers Bureau; Hematology Clínica Universidad de Navarra: Current Employment. Paiva:Adaptive: Honoraria; Takeda: Honoraria, Research Funding; GSK: Honoraria, Research Funding; EngMab: Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Roche Glycart AG: Honoraria, Research Funding; Amgen: Honoraria; Gliead: Honoraria; Oncopeptides: Honoraria. Van De Donk:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding. Martin:Amgen, Johnson & Johnson / Janssen, Sanofi, and Seattle Genetics: Research Funding; GlaxoSmithKline and Legend Biotech: Consultancy; Legend Biotech: Consultancy. Suvannasankha:Regeneron: Research Funding; Sutro Biopharma: Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Janssen Oncology: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Corsale:Janssen R&D: Current Employment. Schecter:Janssen: Current Employment, Current holder of stock options in a privately-held company. De Braganca:Janssen R&D: Current Employment. Jackson:Janssen R&D: Current Employment. Varsos:Janssen: Current Employment. Deraedt:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Roccia:Janssen: Current Employment, Current equity holder in publicly-traded company. Li:Janssen R&D, a Johnson and Johnson company: Current Employment, Current equity holder in publicly-traded company. Zudaire:Janssen R&D, Johnson and Johnson: Current Employment. Pacaud:Legend Biotech USA: Current Employment. Avivi:Novartis: Speakers Bureau; Kite, a Gilead Company: Speakers Bureau. San-Miguel:Abbvie, Amgen, BMS, Celgene, GSK, Haemalogix, Janssen-Cilag, Karyopharm, MSD, Novartis, Takeda, Regeneron, Roche, Sanofi, and SecuraBio: Consultancy, Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal